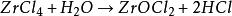Zirconium (IV) chloride, makwaara dị kazirconium tetrachloride, nwere usoro molekụla ZrCl4 yana ịdị arọ molekụla nke 233.04. A na-eji ya eme ihe dị ka reagents nyocha, organic synthesis catalysts, waterproofing agents, tanning agents.
Aha ngwaahịa:Zirconium chlorideZirconium tetrachloride; Zirconium (IV) chloride
Ibu ibu: 233.04
EINECS: 233-058-2
Ebe isi mmiri: 331 (sublimation)
Njupụta: 2.8
Usoro kemịkalụ:ZrCl4
CAS:10026-11-6
Ebe mgbaze:437
Mmiri solubility: Solubility na mmiri oyi
1. Njirimara
Njirimara anụ ahụ na kemịkal
1. Character: White kenkowaputa crystal ma ọ bụ ntụ ntụ, mfe deliquescent.
2. Ebe mgbaze (℃): 437 (2533.3kPa)
3. Ebe esi esi (℃): 331 (sublimation)
4. Njupụta nke ikwu (mmiri = 1): 2.80
5. Nrụgide ikuku juru (kPa): 0.13 (190 ℃)
6. Nsogbu siri ike (MPa): 5.77
7. Solubility: Soluble na mmiri oyi, ethanol, na ether, na-adịghị edozi na benzene, carbon tetrachloride, na carbon disulfide.
Ọ dị mfe ịmịkọrọ mmiri na mmiri, hydrolyzed n'ime hydrogen chloride na zirconium oxychloride n'ime ikuku iru mmiri ma ọ bụ ngwọta mmiri, nhata bụ nke a:
Nkwụsi ike
1. Nkwụsi ike: Stable
2. Ihe ndị a machibidoro iwu: mmiri, amines, alcohols, acids, esters, ketones
3. Ọnọdụ iji zere kọntaktị: iru mmiri ikuku
4. Polymerization ize ndụ: enweghị polymerization
5. ngwaahịa ire ere: chloride
2. Ngwa
(1) A na-eji ya eme ọla zirconium, pigmenti, ihe mkpuchi mmiri textile, ihe na-acha akpụkpọ anụ, wdg.
(2) A na-eji maka mmepụta nke ogige zirconium na ihe ndị na-emepụta ihe na-emepụta ihe na-emepụta ihe, ọ nwere ike iji ya mee ihe dị ka ihe mgbaze na ihe na-eme ka ọ dị ọcha maka metal magnesium gbazere, na mmetụta nke iwepụ ígwè na silicon.
3.Synthetic usoro
Tulee zirconia na carbon oji nke nwere calcined dị ka nha nha nha nha, gwakọta nke ọma ma tinye ha n'ime ụgbọ mmiri poselin. Tinye ụgbọ mmiri poselin n'ime ọkpọkọ poselin wee kpoo ya ruo 500 ℃ n'ime iyi gas chlorine maka nhazi. Na-anakọta ngwaahịa ahụ site na iji ọnyà na okpomọkụ ụlọ. N'ịtụle sublimation nke zirconium tetrachloride na 331 ℃, a 600mm ogologo tube nwere ike iji resblimate ya na hydrogen gas iyi na 300-350 ℃ wepụ oxides na ferric chloride na zirconium chloride.
4. Mmetụta na gburugburu ebe obibi
Ihe egwu ahụike
Ụzọ mbuso agha: iku ume, ingestion, kọntaktị akpụkpọ.
Ihe ize ndụ ahụike: iku ume nwere ike ịkpata mgbakasị iku ume, ilo. Ọ nwere mgbakasị ahụ siri ike ma nwee ike ịkpata ọkụ akpụkpọ na mmebi anya. Nlekọta ọnụ nwere ike ime ka ọkụ dị n'ọnụ na akpịrị, ọgbụgbọ, ọgbụgbọ, stool mmiri, stool ọbara, daa, na mgbakasị ahụ.
Mmetụta na-adịghị ala ala: Na-akpata granuloma akpụkpọ anụ. Mmetụta dị nro na traktị iku ume.
Toxicology na gburugburu ebe obibi
Nnukwu nsi: LD501688mg/kg (nchịkwa ọnụ na oke); 665mg/kg (ọnụ ọnụ òké)
Àgwà ndị dị ize ndụ: Mgbe a na-etinye ya na okpomọkụ ma ọ bụ mmiri, ọ na-erepịa ma na-ahapụ okpomọkụ, na-ahapụ anwụrụ ọkụ na-egbu egbu.
Ngwa ire ere: hydrogen chloride.
Usoro nyocha ụlọ nyocha: Plasma spectroscopy (usoro NIOSH 7300)
Mkpebi na ikuku: na-anakọta ihe nlele ahụ na nzacha, gbazee ya na acid, wee jiri spectroscopy absorption spectroscopy tụọ ya.
Ụkpụrụ gburugburu ebe obibi: Nchekwa Ọrụ na nlekọta ahụike (1974), Nkezi Ogologo Oge Ikuku 5.
Nzaghachi mberede nke ntapu
Kewapụ ebe a na-emetọ mmiri, guzobe akara ịdọ aka ná ntị gburugburu, ma tụọ aro ndị ọrụ ọgwụgwọ mberede ka ha yi mkpuchi gas na uwe nchebe kemịkal. Abatala kọntaktị ozugbo na ihe ndị a na-agbapụta, zere uzuzu, jiri nlezianya kpochaa ya, dozie ihe ngwọta nke ihe dịka 5% mmiri ma ọ bụ acid, jiri nwayọọ nwayọọ tinye mmiri amonia na-agbaze ruo mgbe mmiri ozuzo na-ada, wee tụfuo ya. Ị nwekwara ike ịsacha ya na nnukwu mmiri, ma gbanye mmiri ịsa ahụ n'ime usoro mmiri mkpofu. Ọ bụrụ na enwere nnukwu ntanye, wepụ ya n'okpuru nduzi nke ndị ọrụ nka. Ụzọ mkpofu mkpofu: Gwakọta ihe mkpofu na sodium bicarbonate, fesa mmiri amonia, tinyekwa ice e gwepịara egwepịa. Mgbe mmeghachi omume ahụ kwụsịrị, jiri mmiri kpochaa n'ime ọwa mmiri.
Usoro nchebe
Nchedo iku ume: Mgbe ekpughere ya na uzuzu, ekwesịrị iyi ihe mkpuchi gas. Yiri ngwa iku ume nke nwere onwe ya mgbe ọ dị mkpa.
Nchedo anya: Yiri enyo nchekwa kemịkal.
Uwe nchebe: Yiri uwe ọrụ (nke ihe mgbochi corrosion mere).
Nchedo aka: Yiri gloves roba.
Ndị ọzọ: Mgbe ọrụ gasịrị, saa ahụ ma gbanwee uwe. Chekwaa uwe ndị nwere nsí dị iche iche wee jiri ya mee ihe mgbe ịsachara ya. Nọgide na-enwe ezi àgwà ịdị ọcha.
Usoro enyemaka mbụ
Mmekọahụ akpụkpọ: ozugbo were mmiri sachaa maka opekata mpe nkeji iri na ise. Ọ bụrụ na enwere ọkụ, chọọ ọgwụgwọ.
Nleta anya: Welite nku anya ozugbo wee jiri mmiri na-asọ asọ ma ọ bụ saline physiological sachaa maka opekata mpe nkeji iri na ise.
Inhalation: Wepụ ngwa ngwa site na ebe ahụ gaa n'ebe ikuku dị ọhụrụ. Jikwaa traktị iku ume na-enweghị mgbochi. Mee ume iku ume ma ọ bụrụ na ọ dị mkpa. Chọọ nlekọta ahụike.
Ingestion: Mgbe onye ọrịa tetara, sachaa ọnụ ya ozugbo, emela ka agbọ agbọ, ṅụọ mmiri ara ehi ma ọ bụ akwa ọcha. Chọọ nlekọta ahụike.
Ụzọ ọkụ ọkụ: ụfụfụ, carbon dioxide, ájá, akọrọ ntụ ntụ.
5. Usoro nchekwa
Chekwaa n'ụlọ nkwakọba ihe dị jụụ, kpọrọ nkụ ma nwee ikuku nke ọma. Wepụ n'ọkụ ọkụ na isi mmalite okpomọkụ. A ghaghị mechie nkwakọ ngwaahịa ahụ ma chebe ya pụọ na mmiri mmiri. Ekwesịrị ịchekwa ya iche na acid, amines, alcohols, esters, wdg, ma zere ịkwakọba nchekwa. Ebe nchekwa ahụ kwesịrị ịkwado ihe ndị kwesịrị ekwesị iji nwee ntapu.
6 na-edezi data chemistry
1. Ntụaka uru maka hydrophobic parameter ngụkọta oge (XlogP): Ọ dịghị
2. Ọnụọgụ nke ndị na-enye njikọ njikọ hydrogen: 0
3. Ọnụọgụ nke ndị nnabata hydrogen: 0
4. Ọnụọgụ nke njikọ kemịkalụ rotatable: 0
5. Ọnụ ọgụgụ ndị na-akpụ akpụ: Ọ dịghị
6. Topological molecule polarity surface area: 0
7. Ọnụọgụ nke atọm arọ: 5
8. Ụgwọ elu: 0
9. Mgbagwoju anya: 19.1
10. Ọnụọgụ nke Atọm Isotope: 0
11. Kpebisie ike na ọnụ ọgụgụ nke atọm Ọdịdị center: 0
12. Ọnụọgụ ebe a na-ewu ụlọ atọm na-ejighị n'aka: 0
13. Kpebie ọnụọgụ kemịkalụ stereo center: 0
14. Ọnụọgụ nke stereocenter kemịkalụ na-ejighị n'aka: 0
15. Ọnụọgụ nke covalent bond units: 1
Oge nzipu: Mee-25-2023